8 (4) A, C 0.1 gm of organic compound was analysed by Kjeldahl's method. In analysis produced NH, absorbed in 30 ml N/5 H,SO. The remaining acid required 20 ml N/10 NaOH
Por um escritor misterioso
Last updated 16 junho 2024

Click here:point_up_2:to get an answer to your question :writing_hand:84 a c01 gm of organic compound was analysed bykjeldahls method in analysis produced nhabsorbed

29) 0.2 g of an organic compound was analysed by Kjedahl's method. Ammonia evolved was absorbed an 60 mL N/5 H2SO4. Unused acid required 40 mL of N/10 NaOH complete neutralisation. Find
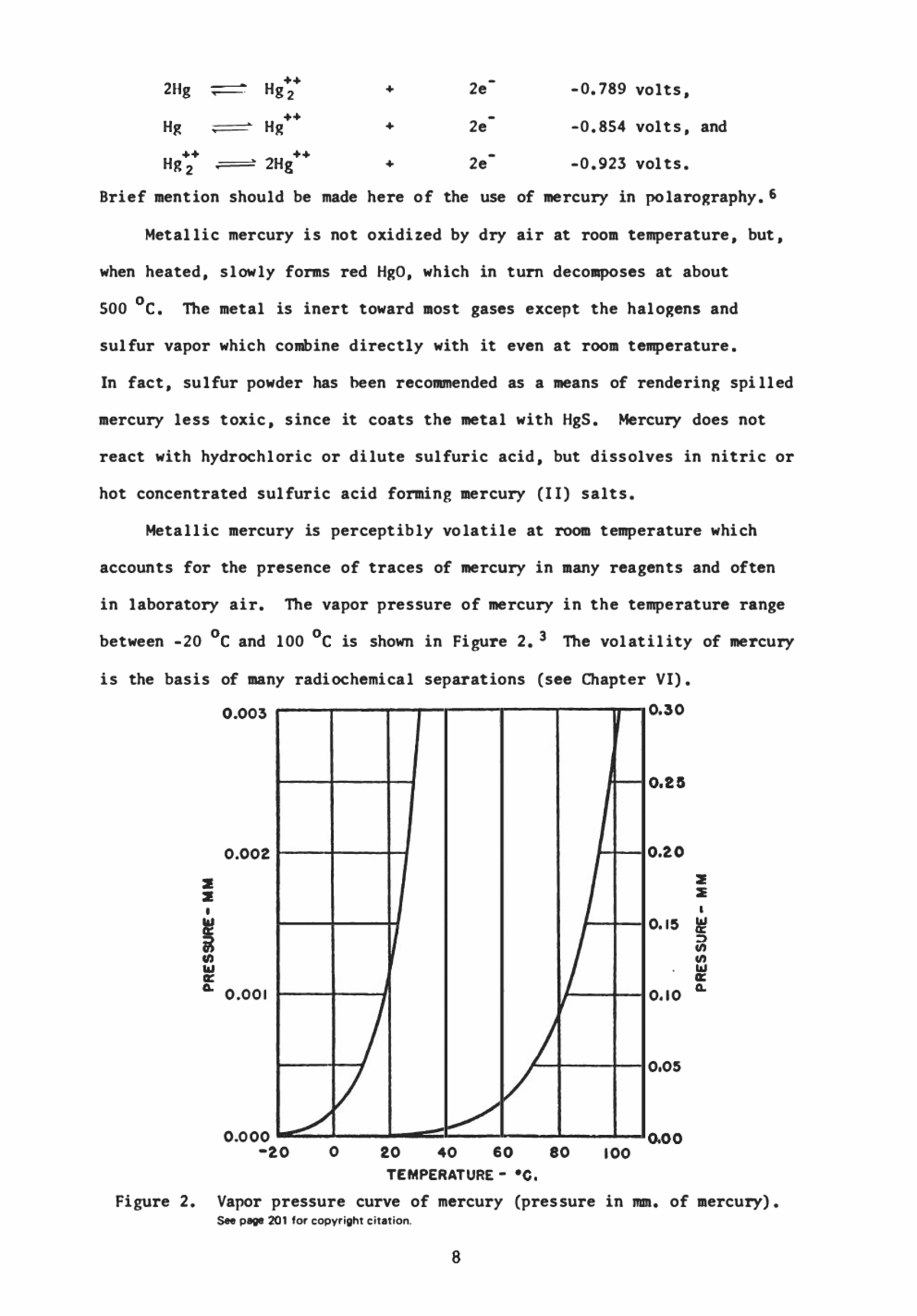
REVIEW OF FEATURES OF MERCURY CHEMISTRY OF CHIEF INTEREST TO RADIOCHEMISTS, Radiochemistry of Mercury

Nitrogen - ScienceDirect

0.2 gm of an organic compound was analysed by kjeldahl's method the ammonia evolved was absorbed

NR. Ammonia obtained from 0.4 g of an organic substance by Kjeldahl's method was absorbed in 30 ml. of 0-25 MH SO. The excess of the acid was neutralized by the addition


The Titration in the Kjeldahl Method of Nitrogen Determination: Base or Acid as Titrant?

0.2 g of an organic compound was analysed by Kjeldahl's method. Ammonia evolved was absorbed in 60 ml N/5

PDF) 242 Water, Nutrient, and Acid Requirements for Crops Grown Hydroponically in a Celss

0.2 gm of an organic compound was analysed by kjeldahl's method the ammonia evolved was absorbed

The Titration in the Kjeldahl Method of Nitrogen Determination: Base or Acid as Titrant?

Estequiometria quimica, Ejercicios de Química

0 156 gram of an organic compound on Kjeldahl's analysis produced NH3 which was absorbed in 60 25ml of 0 - Chemistry - Organic Chemistry Some Basic Principles and Techniques - 13768967

Estequiometria quimica, Ejercicios de Química
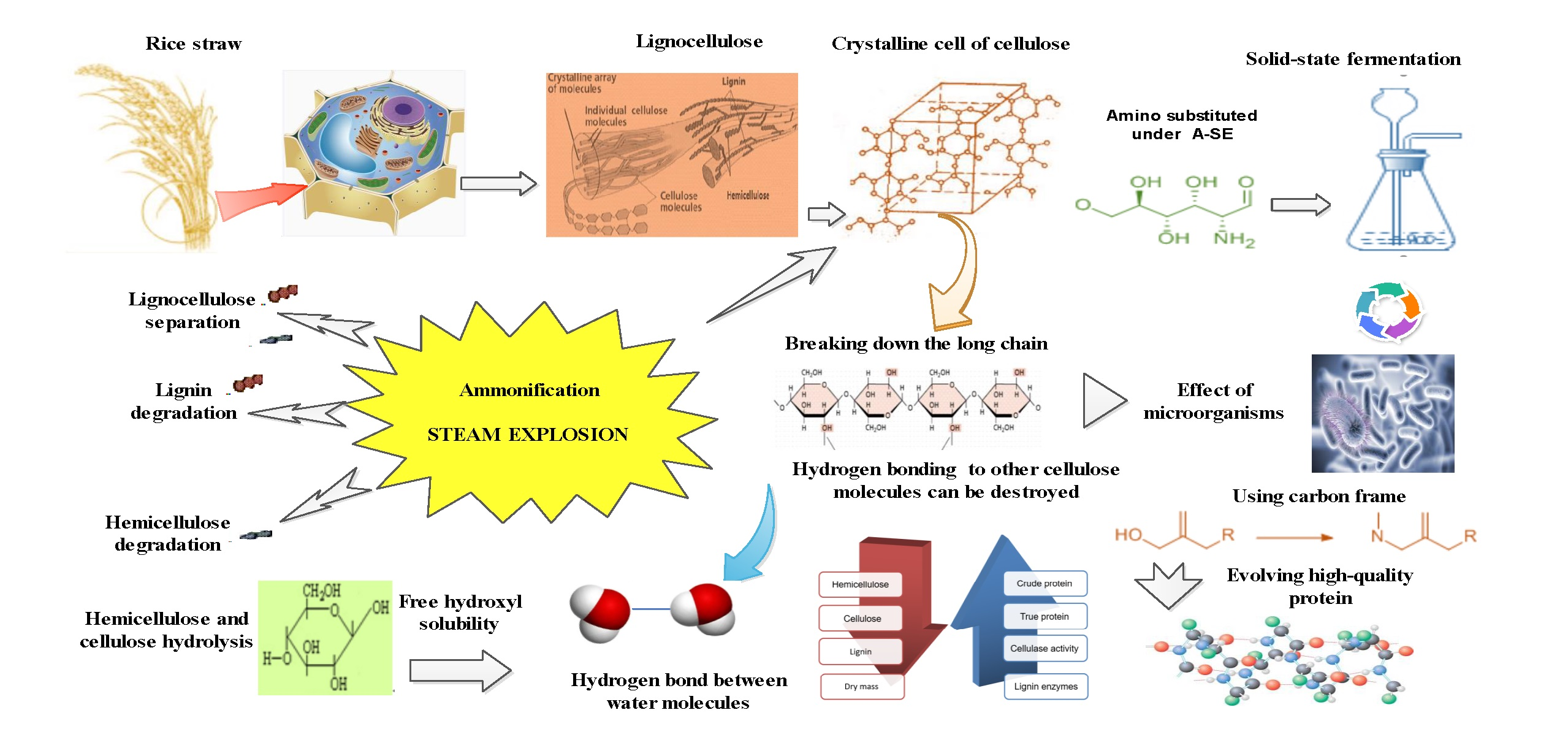
Sustainability, Free Full-Text
Recomendado para você
-
 Analyse or Analyze: How to Use Each Correctly16 junho 2024
Analyse or Analyze: How to Use Each Correctly16 junho 2024 -
 Analysed LLC (en)16 junho 2024
Analysed LLC (en)16 junho 2024 -
 analysed Archives - The Oxford Review - OR Briefings16 junho 2024
analysed Archives - The Oxford Review - OR Briefings16 junho 2024 -
Analysed databases. Source. Web of Science Core Collection (2022).16 junho 2024
-
 Gail Carriger Quote: “Silence descended while they all analysed her words for hidden meaning. They were lost because there was none. Simplicit”16 junho 2024
Gail Carriger Quote: “Silence descended while they all analysed her words for hidden meaning. They were lost because there was none. Simplicit”16 junho 2024 -
 The Ultimate UCAS Personal Statement by Agarwal, Rohan16 junho 2024
The Ultimate UCAS Personal Statement by Agarwal, Rohan16 junho 2024 -
 Analysed Images16 junho 2024
Analysed Images16 junho 2024 -
 AI governance challenges and UK approach analysed in govt report16 junho 2024
AI governance challenges and UK approach analysed in govt report16 junho 2024 -
 Sergio Reguilón's stop-start Manchester United career analysed16 junho 2024
Sergio Reguilón's stop-start Manchester United career analysed16 junho 2024 -
Bridge Eigen Modes analysed with Karamba on Vimeo16 junho 2024
você pode gostar
-
 Knocked Loose Mistakes Like Fractures Official Music Video on Make a GIF16 junho 2024
Knocked Loose Mistakes Like Fractures Official Music Video on Make a GIF16 junho 2024 -
hmm.updates. (BOOK 1) (OLD) - Sonic.Exe VS Metal Sonic - Wattpad16 junho 2024
-
 Chainsaw Man Episode 1-12End Japanese Anime DVD English16 junho 2024
Chainsaw Man Episode 1-12End Japanese Anime DVD English16 junho 2024 -
 PLEASE READ IF YOU OWN A LOW TIER HALO, PLEASE!16 junho 2024
PLEASE READ IF YOU OWN A LOW TIER HALO, PLEASE!16 junho 2024 -
 LOPEZ XG16 junho 2024
LOPEZ XG16 junho 2024 -
 Sinal de aviso amarelo - Estrada áspera à frente - Isolado fotos16 junho 2024
Sinal de aviso amarelo - Estrada áspera à frente - Isolado fotos16 junho 2024 -
 Tower of Fantasy: Black and Gold Nucleus locations on Astra16 junho 2024
Tower of Fantasy: Black and Gold Nucleus locations on Astra16 junho 2024 -
 Drama Total : Meus Personagens 6 (Remake) by Desconekitado on16 junho 2024
Drama Total : Meus Personagens 6 (Remake) by Desconekitado on16 junho 2024 -
 Tonikaku Kawaii 2 Temporada – Dublado - Episódio 6 - Animes Online16 junho 2024
Tonikaku Kawaii 2 Temporada – Dublado - Episódio 6 - Animes Online16 junho 2024 -
 Selecione 3 bolas de sinuca e coloque nos círculos16 junho 2024
Selecione 3 bolas de sinuca e coloque nos círculos16 junho 2024
