FDA allows Houston cancer doctor to resume drug trial
Por um escritor misterioso
Last updated 18 maio 2024

Federal regulators have lifted a partial hold on a clinical trial performed by Stanislaw

FDA issues warning to controversial Houston cancer doctor

Doctor claims to cure pediatric cancer, critics skeptical
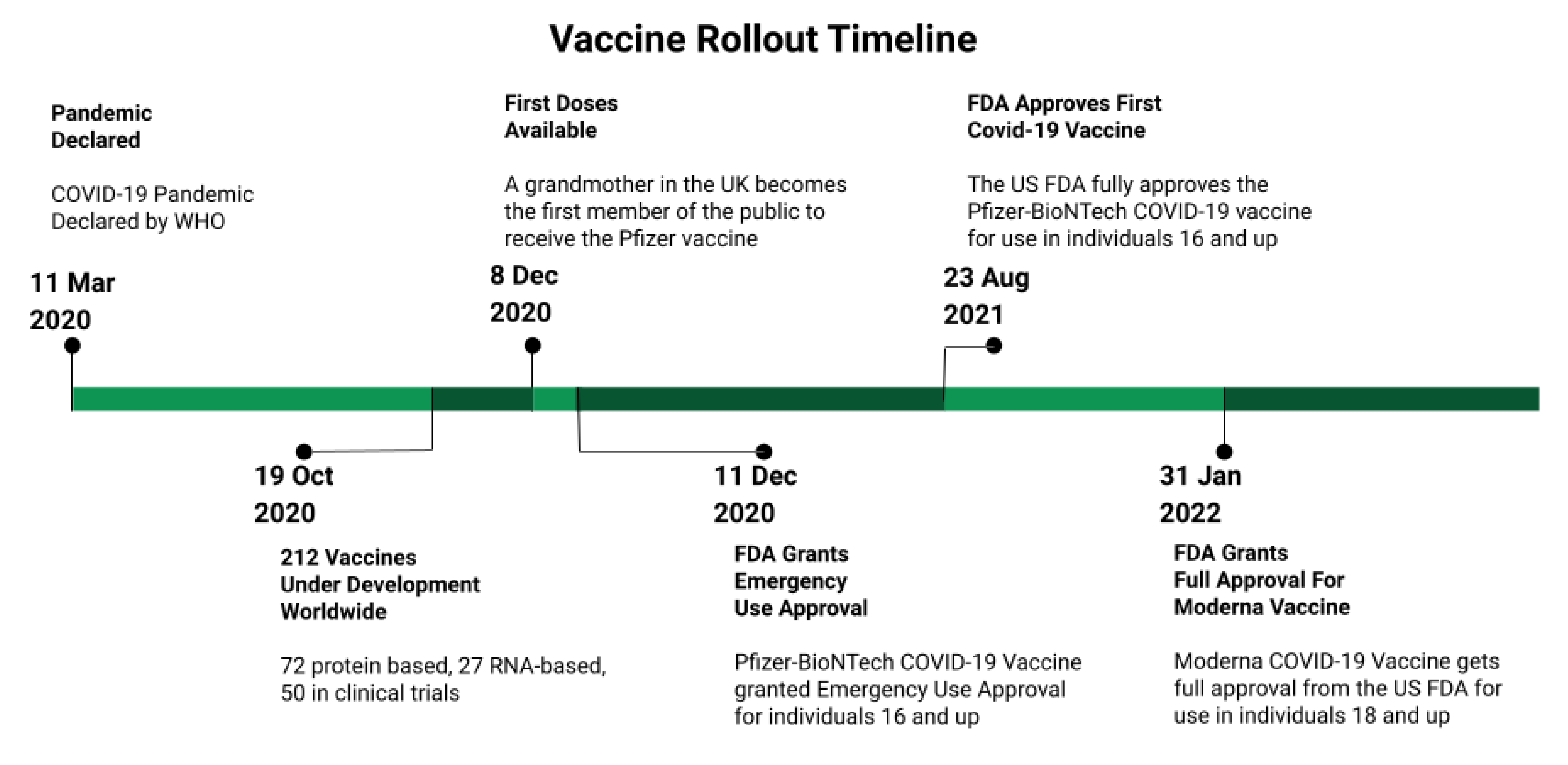
Vaccines, Free Full-Text
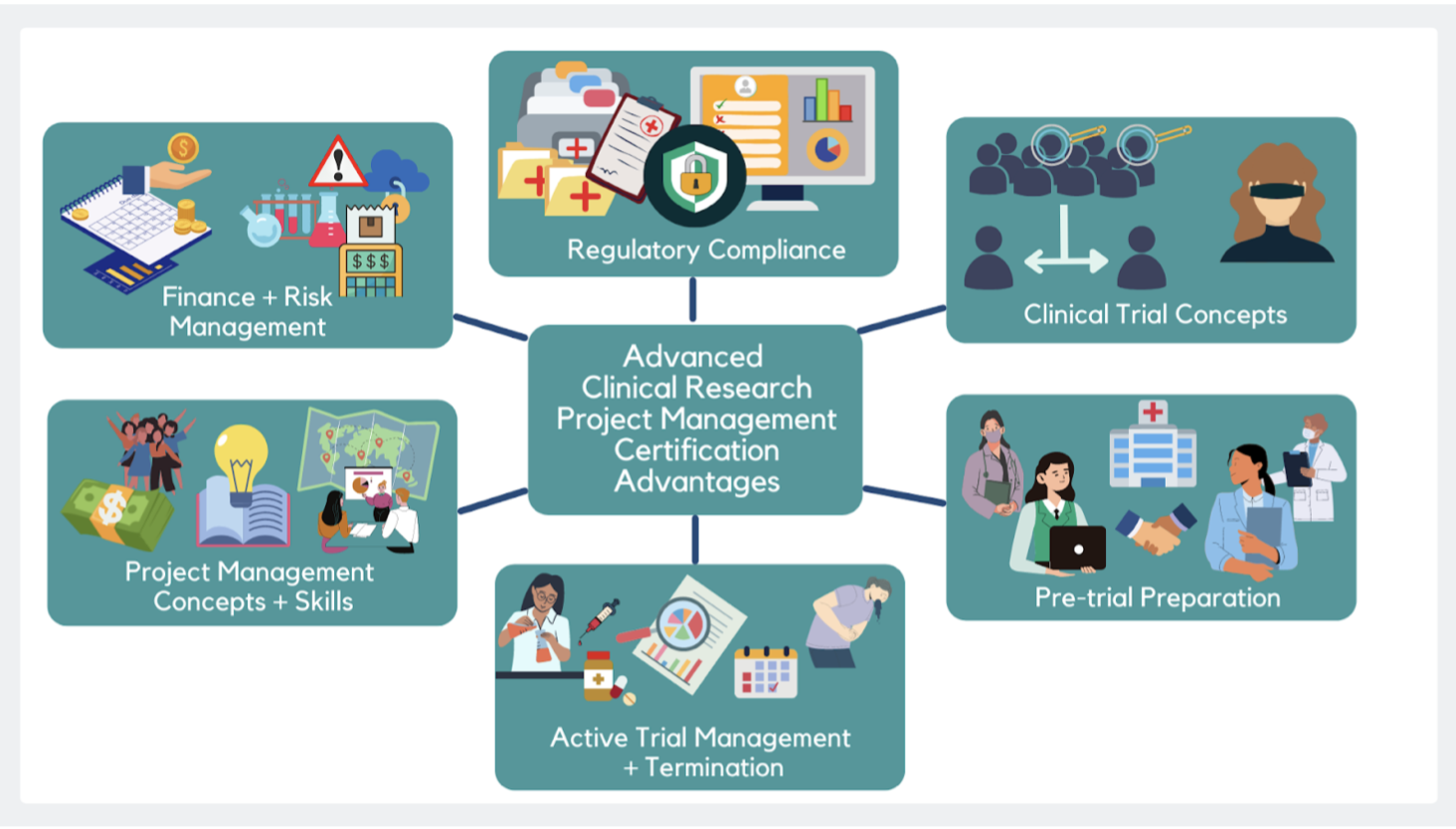
Clinical Research Project Manager - CCRPS

Katy Rezvani MD Anderson Cancer Center

Fast-Track Drug Approval, Designed for Emergencies, Is Now Routine - WSJ
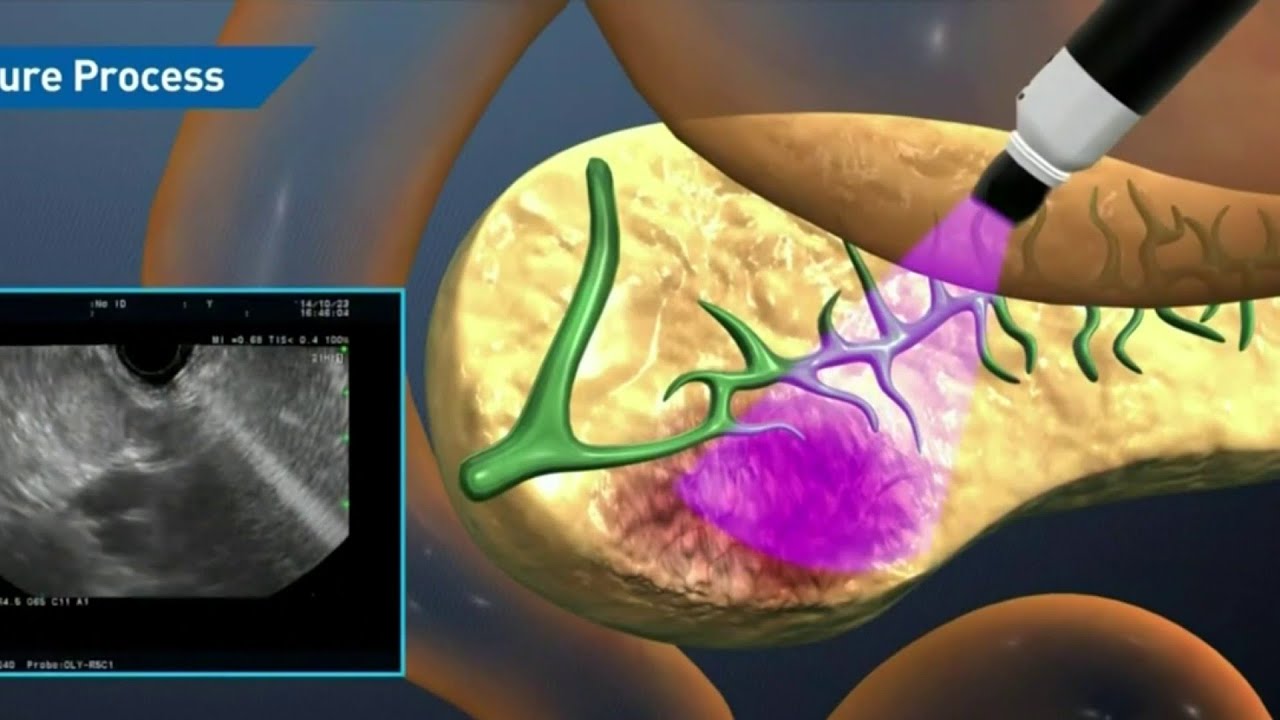
Houston doctor discovers way to treat pancreatic cancer during clinical trial

Elias Jabbour MD Anderson Cancer Center

Radiotherapy and paclitaxel plus pazopanib or placebo in anaplastic thyroid cancer (NRG/RTOG 0912): a randomised, double-blind, placebo-controlled, multicentre, phase 2 trial - The Lancet Oncology
Recomendado para você
-
 Younan Nowzaradan - Age, Bio, Birthday, Family, Net Worth18 maio 2024
Younan Nowzaradan - Age, Bio, Birthday, Family, Net Worth18 maio 2024 -
Dr. Now, Conservative Hero18 maio 2024
-
Celso Zucatelli encontra médico de Quilos Mortais nos Estados18 maio 2024
-
 Younan Nowzaradan, MD, 4009 Bellaire Blvd, Ste K, Houston, TX, Physicians' office, including specialists - MapQuest18 maio 2024
Younan Nowzaradan, MD, 4009 Bellaire Blvd, Ste K, Houston, TX, Physicians' office, including specialists - MapQuest18 maio 2024 -
 Contact18 maio 2024
Contact18 maio 2024 -
 My 600-Lb. Life': Mike Loses Custody If He Does Dr. Now's Program – Hollywood Life18 maio 2024
My 600-Lb. Life': Mike Loses Custody If He Does Dr. Now's Program – Hollywood Life18 maio 2024 -
 My 600-Lb Life': Shannon Lowery Struggling With Move to Houston for Surgery With Dr. Now18 maio 2024
My 600-Lb Life': Shannon Lowery Struggling With Move to Houston for Surgery With Dr. Now18 maio 2024 -
 My 600-lb Life' Dolly Martinez Chooses Homeless Deaf Boyfriend Over Her Health!18 maio 2024
My 600-lb Life' Dolly Martinez Chooses Homeless Deaf Boyfriend Over Her Health!18 maio 2024 -
 The giver – Dentistry News18 maio 2024
The giver – Dentistry News18 maio 2024 -
 Weightless Excerpt: My 600-lb Life Needs To Be Canceled18 maio 2024
Weightless Excerpt: My 600-lb Life Needs To Be Canceled18 maio 2024
você pode gostar
-
Melhores jogos indie – abril de 201818 maio 2024
-
 The Rising of the Shield Hero RERISE cierra sus puertas definitivamente18 maio 2024
The Rising of the Shield Hero RERISE cierra sus puertas definitivamente18 maio 2024 -
 Geleia De Morango Susin Chimia Colonial - Serra Gaúcha 710g18 maio 2024
Geleia De Morango Susin Chimia Colonial - Serra Gaúcha 710g18 maio 2024 -
 Google Play Store 7.8.16 APK Download by Google LLC - APKMirror18 maio 2024
Google Play Store 7.8.16 APK Download by Google LLC - APKMirror18 maio 2024 -
Grau Stunt Wheelie Bikes M X APK (Android Game) - Free Download18 maio 2024
-
 64 Desenhos da Turma da Mônica para Colorir18 maio 2024
64 Desenhos da Turma da Mônica para Colorir18 maio 2024 -
 Assistir Peter Grill to Kenja no Jikan 2 Episodio 8 Online18 maio 2024
Assistir Peter Grill to Kenja no Jikan 2 Episodio 8 Online18 maio 2024 -
New Rell Seas Bisento And Ship Battles Sneak! #robloxanime #viral #vir18 maio 2024
-
 DVD Anime Yofukashi no Uta / Call of the Night English Dubbed Free Shipping18 maio 2024
DVD Anime Yofukashi no Uta / Call of the Night English Dubbed Free Shipping18 maio 2024 -
 Age of Marvel!: Marvel's Wardrobe: O Poderoso Thor18 maio 2024
Age of Marvel!: Marvel's Wardrobe: O Poderoso Thor18 maio 2024



