Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Last updated 19 junho 2024

Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

IHEP (International Hepatology Education Program)
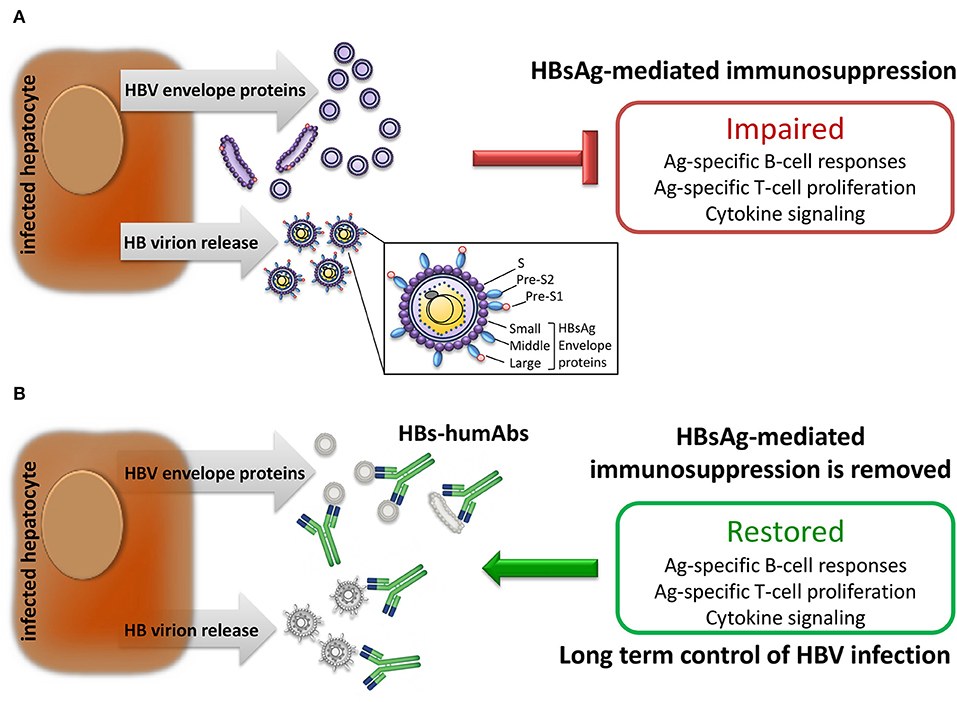
Frontiers Human Monoclonal Antibodies as Adjuvant Treatment of

HBV replication inhibitors. - Abstract - Europe PMC

Bayer taps Berkeley Lights to automate drug discovery

Therapeutic vaccination for treatment of chronic hepatitis B

Landon Loving sur LinkedIn : GSK's $100M ADC bet in doubt after

Heplisav-B: A New Hepatitis B Vaccine That Can Be Used For Pre

Landon Loving on LinkedIn: Fierce Biotech Fundraising Tracker '23

Assembly Bio breaks off hepatitis B deal with Antios Therapeutics
EuroBiotech Report: Woodford's loss, BioInvent's major failure
Recomendado para você
-
![Halt [DOORS] by TheWandererH on DeviantArt](https://images-wixmp-ed30a86b8c4ca887773594c2.wixmp.com/f/f5e9214b-46cf-4479-bac6-881ddf00ec6e/dfece3t-94478477-1c07-408b-ad12-407e1699f74a.jpg/v1/fill/w_1280,h_1114,q_75,strp/halt__doors__by_thewandererh_dfece3t-fullview.jpg?token=eyJ0eXAiOiJKV1QiLCJhbGciOiJIUzI1NiJ9.eyJzdWIiOiJ1cm46YXBwOjdlMGQxODg5ODIyNjQzNzNhNWYwZDQxNWVhMGQyNmUwIiwiaXNzIjoidXJuOmFwcDo3ZTBkMTg4OTgyMjY0MzczYTVmMGQ0MTVlYTBkMjZlMCIsIm9iaiI6W1t7ImhlaWdodCI6Ijw9MTExNCIsInBhdGgiOiJcL2ZcL2Y1ZTkyMTRiLTQ2Y2YtNDQ3OS1iYWM2LTg4MWRkZjAwZWM2ZVwvZGZlY2UzdC05NDQ3ODQ3Ny0xYzA3LTQwOGItYWQxMi00MDdlMTY5OWY3NGEuanBnIiwid2lkdGgiOiI8PTEyODAifV1dLCJhdWQiOlsidXJuOnNlcnZpY2U6aW1hZ2Uub3BlcmF0aW9ucyJdfQ.EmHkO-1vnNgie3PrRzW_Ip0K030MAIq6lvZbEgWdsXQ) Halt [DOORS] by TheWandererH on DeviantArt19 junho 2024
Halt [DOORS] by TheWandererH on DeviantArt19 junho 2024 -
 UncreativeCJ on Tumblr19 junho 2024
UncreativeCJ on Tumblr19 junho 2024 -
What the heck!19 junho 2024
-
 Halt doors Minecraft Skin19 junho 2024
Halt doors Minecraft Skin19 junho 2024 -
 The Free-College Fantasy19 junho 2024
The Free-College Fantasy19 junho 2024 -
 LED Tail Light Set, Early Bronco19 junho 2024
LED Tail Light Set, Early Bronco19 junho 2024 -
 bbe23ddf8ed07d3c90b473027e9142583c759486.gif19 junho 2024
bbe23ddf8ed07d3c90b473027e9142583c759486.gif19 junho 2024 -
 How Ghost Kitchens Took Over America's Restaurants19 junho 2024
How Ghost Kitchens Took Over America's Restaurants19 junho 2024 -
 Doors fnf vs halt 1 Project by Sleet Porter19 junho 2024
Doors fnf vs halt 1 Project by Sleet Porter19 junho 2024 -
 Doors Entities Figure Rush Ambush A-60 A-90 A-120 Seek Stare El Goblino Dupe Halt Jeff Bob Eyes Screech Jack Glitch Timothy Shadow Window | Art Print19 junho 2024
Doors Entities Figure Rush Ambush A-60 A-90 A-120 Seek Stare El Goblino Dupe Halt Jeff Bob Eyes Screech Jack Glitch Timothy Shadow Window | Art Print19 junho 2024
você pode gostar
-
 Meu Contador Online - Certificado Digital Serasa19 junho 2024
Meu Contador Online - Certificado Digital Serasa19 junho 2024 -
 Jack Black 'Super Mario Bros.' Ballad 'Peaches': Hot 100 First-Timers – Billboard19 junho 2024
Jack Black 'Super Mario Bros.' Ballad 'Peaches': Hot 100 First-Timers – Billboard19 junho 2024 -
 Used Fmx 540 for sale. Agco equipment & more19 junho 2024
Used Fmx 540 for sale. Agco equipment & more19 junho 2024 -
 Jogos Para Meninos Ate 4 Anos: Promoções19 junho 2024
Jogos Para Meninos Ate 4 Anos: Promoções19 junho 2024 -
project playtime gratis|TikTok Search19 junho 2024
-
 Honkai Star Rail Leaks - Xueyi Skills and Abilities19 junho 2024
Honkai Star Rail Leaks - Xueyi Skills and Abilities19 junho 2024 -
 Zelda's Lullaby - Flute + Big Band Version! ft. Sharpeye (The 8-Bit Big Band)19 junho 2024
Zelda's Lullaby - Flute + Big Band Version! ft. Sharpeye (The 8-Bit Big Band)19 junho 2024 -
 Create a ASTD ORBS 7/12/21 Tier List - TierMaker19 junho 2024
Create a ASTD ORBS 7/12/21 Tier List - TierMaker19 junho 2024 -
 Respect Dracule Hawk-Eye Mihawk (Netflix's One Piece, Live19 junho 2024
Respect Dracule Hawk-Eye Mihawk (Netflix's One Piece, Live19 junho 2024 -
 Evento Empresarial - Team Building - Fotografia em Evento …19 junho 2024
Evento Empresarial - Team Building - Fotografia em Evento …19 junho 2024

